IGALMI™ (Dexmedetomidine): First Sublingual Medication FDA Approved for Acute Agitation
Today FDA has approved the first sublingual medication, IGALMI (Dexmedetomidine) for the management of acute agitation in indications discussed below. We have summarized this medication in the following sections:
- Indication
- Mechanism of Action
- Recommended DOse
- Important Recommendations Before Initiating & During Treatment.
- What to do if agitation persists after first dose?
- Important Warnings & Precautions.
- Drug Interactions
INDICATION:
Acute treatment of agitation associated with
- Schizophrenia (adults) or
- Bipolar I or II disorder (adults)
MECHANISM OF ACTION:
- Presynaptic alpha-2 adrenergic receptor agonism.
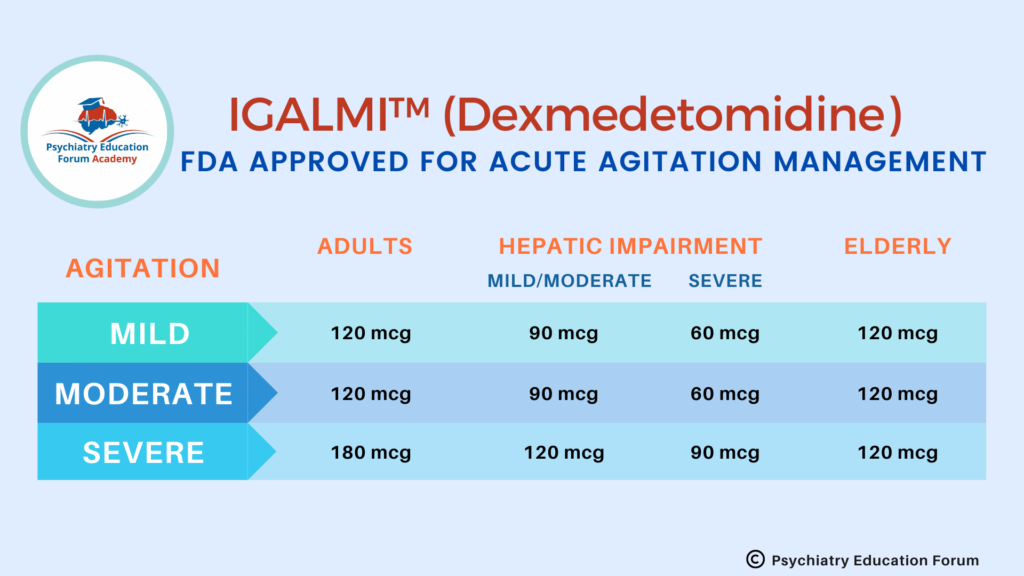
RECOMMENDED DOSE:
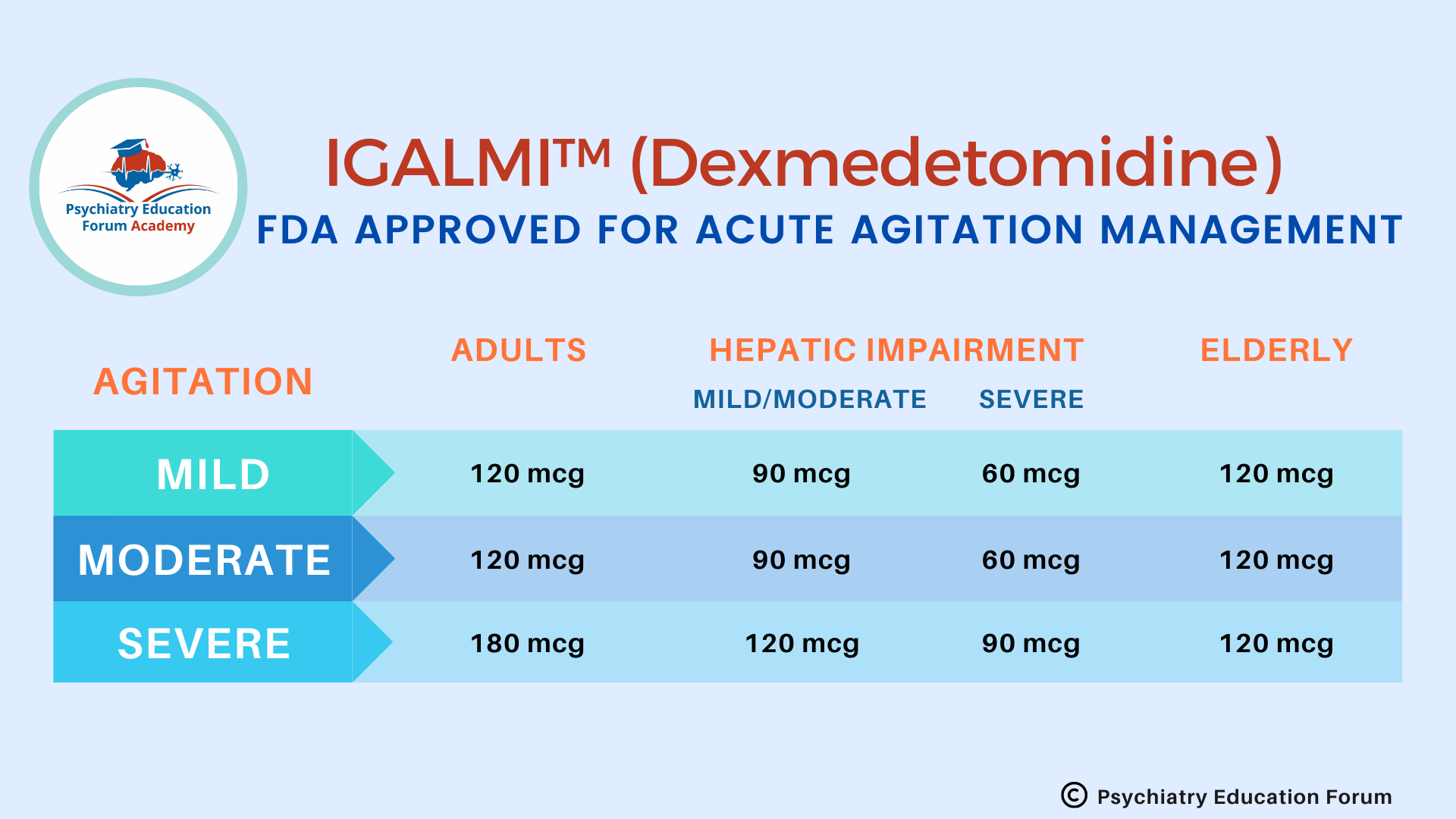
IMPORTANT RECOMMENDATION BEFORE INITIAITNG & DURING TREATMENT:
- IGALMI should be administered under the supervision of a healthcare provider.
- Healthcare provider should monitor vital signs and alertness after administration to prevent falls and syncope.
- Do not chew or swallow IGALMI.
- Do not eat or drink for at least 15 minutes after sublingual administration, or at least one hour after buccal
administration.
WHAT TO DO IF AGITATION PERSISTS AFTER FIRST DOSE?
- up to two additional doses may be administered at least two hours apart.
- Assess vital signs including orthostatic measurements prior to the administration of any subsequent doses.
AVOID ADDITIONAL DOSES IF:
- Systolic blood pressure (SBP) < 90 mmHg
- Diastolic blood pressure (DBP) < 60 mmHg
- Heart rate < 60 beats per minute, or
- Postural decrease in SBP ≥ 20 mmHg or in DBP ≥ 10 mmHg.
IMPORTANT WARNINGS & PRECAUTIONS:
(1) Hypotension, Orthostatic Hypotension, and Bradycardia:
- Avoid in patients with hypotension, orthostatic hypotension, advanced heart block, severe ventricular dysfunction, or history of syncope.
- Ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation.
- Because IGALMI decreases sympathetic nervous system activity, hypotension and/or bradycardia may be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in geriatric patients
(2) QT Interval Prolongation:
- avoid use in patients with risk factors for prolonged QT interval.
(3) Somnolence:
- Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or operating hazardous machinery for at least eight hours after taking IGALMI.
DRUG INTERACTIONS:
(1) Drugs that Prolong the QT interval.
(2) Anesthetics, Sedatives, Hypnotics, Opioids:
- may cause enhanced CNS depressant effects.
- Reduction in dosage of IGALMI or the concomitant medication may be required.
INTERESTED IN LEARNING MORE?
JOIN PSYCHIATRY EDUCATION FORUM ACADEMY MEMBERSHIP
This is a closed membership for medical professionals only.
- 30+ Courses/Sections: Each chapter within these sections is of direct clinical relevance for your daily practice.
- Journal Club: we will post the most recently published psychiatry articles relevant to your daily clinical practice.
- Coffee Club: contain short discussions with clinical experts in the field of psychiatry.
- Essentials of Inpatient Psychiatry Book: All chapters from this book are included in the academy sections.
- Discussion Forum & Community: Connect with other medical professionals and discuss your difficult-to-treat clinical cases.
- Conference Discounts: Academy members get discounted access at our conferences.
- Goal: is to have all important clinically relevant topics in one place for ease of access.








