FDA Eliminates Clozapine REMS Program: What This Means for Patients and Prescribers

In a major regulatory update, the U.S. Food and Drug Administration (FDA) has announced the discontinuation of the Risk Evaluation and Mitigation Strategy (REMS) program for clozapine. Starting February 24, 2025, healthcare providers and pharmacies will no longer need to submit absolute neutrophil count (ANC) results before prescribing or dispensing clozapine. This change aims to improve access to this essential medication for patients with treatment-resistant schizophrenia and reduce the administrative burden on clinicians
🔔 Why Was REMS Required for Clozapine?
Clozapine has long been known for its effectiveness in treatment-resistant schizophrenia, but its use has been restricted due to the risk of severe neutropenia, a condition in which a dangerously low neutrophil count increases infection risk. To monitor this risk, the FDA required prescribers and pharmacies to enroll in the Clozapine REMS program, ensuring that patients underwent routine blood tests before receiving their medication.
Over time, emerging evidence and clinical practice patterns suggested that while blood monitoring remains important, the REMS structure may not be essential for managing neutropenia risk. This led the FDA to reevaluate the program, culminating in its complete removal in 2025.
🧠 What This Change Means for Patients and Providers
The FDA’s decision does not mean that ANC monitoring is unnecessary—rather, it is shifting responsibility back to clinicians to determine the best monitoring practices for their patients. While the REMS program will no longer be enforced, current prescribing guidelines still recommend regular ANC checks based on patient history and risk factors.
Importantly, clozapine’s prescribing information will continue to highlight the risk of severe neutropenia, including the presence of a Boxed Warning. This ensures that both prescribers and patients remain aware of potential risks, even without the formal REMS structure.
🚀 The Role of Advocacy and Clinical Perspectives
The decision to remove REMS requirements followed extensive input from clinicians, patient advocacy groups, and researchers. In late 2024, an FDA advisory committee meeting was held to assess whether the REMS program was still necessary. The outcome of this discussion significantly influenced the agency’s final decision.
Advocacy organizations, including the Schizophrenia & Psychosis Action Alliance, have welcomed the change, citing the reduction of unnecessary barriers to clozapine access. Many clinicians also support the move, as it streamlines prescribing while maintaining essential patient safety measures.
👉 Next Steps for Clinicians and Patients
As this transition takes place, the FDA will work with manufacturers to update clozapine’s prescribing guidelines. In the meantime, patients and prescribers should continue following ANC monitoring recommendations as outlined in clinical guidelines.
This policy update marks a significant step in reducing treatment barriers while ensuring the safe and effective use of clozapine. Providers should stay informed about the latest monitoring recommendations to optimize patient outcomes.
Reference:
- FDA Update (link)
FOR ACADEMY MEMBERS:
PSYCHIATRY EDUCATION FORUM ACADEMY'S
MANAGING CLOZAPINE ADVERSE EVENTS
UNDERSTANDING THE BASICS & BEYOND
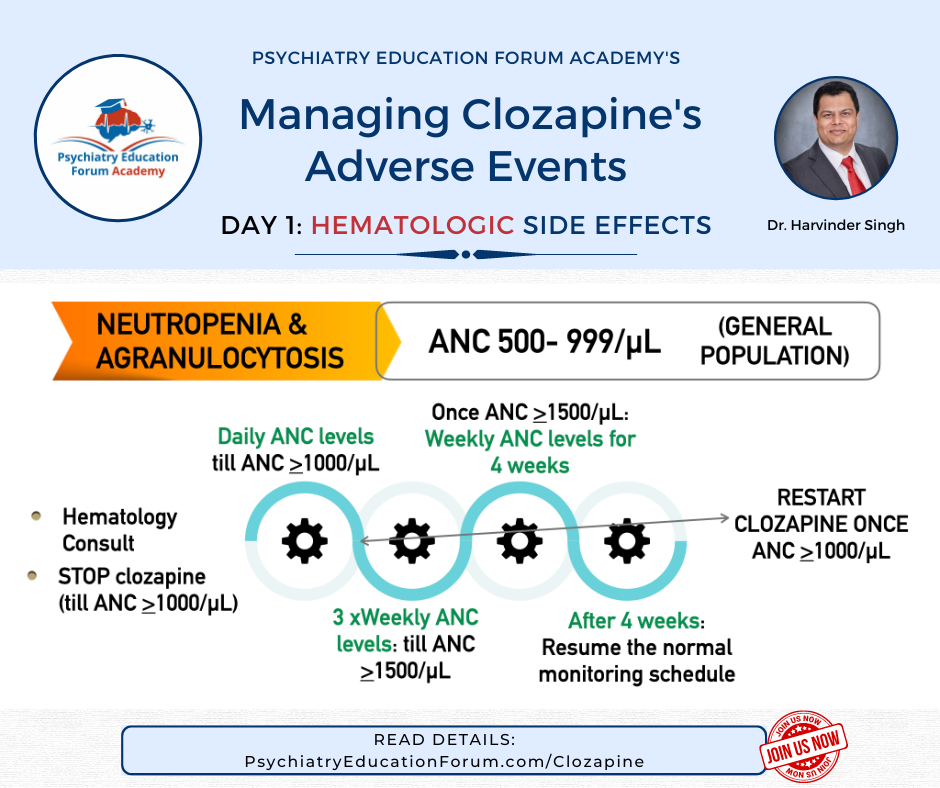
Here is one of the clinically relevant slide from this discussion:
MANAGEMENT OPTION FOR: ANC 500-999/uL DURING CLOZAPINE TREATMENT:
FOR ACADEMY MEMBERS ONLY
INTERESTED IN ACCESS TO THIS & OTHER CLINICALLY RELEVANT LECTURE SERIES?
JOIN ACADEMY MEMBERSHIP:
This is a closed membership for medical professionals only.
- 400+ Clinically Relevant Chapters: Each chapter within these sections is of direct clinical relevance for your daily practice. (Table of Content)
- Journal Club: we will post the most recently published psychiatry articles relevant to your daily clinical practice. (Read Content)
- Clinical Case Discussion: Dr. Singh (Psychiatry) and Dr. Kaur (Family Medicine) discuss clinical cases to integrate the clinical cases from Psychiatry and Medicine. (Read Content)
- Monthly Insights: Gain access to our monthly sessions featuring the latest on recent publications, new medication approvals, FDA updates, and more. (Monthly Insights)
- Discussion Forum & Community: Connect with other medical professionals and discuss your difficult-to-treat clinical cases. (Academy Network)
- Goal: is to have all important clinically relevant topics in one place for ease of access.
DISCOUNTS AVAILABLE FOR: Residents & Students ONLY.
Email us your student information (program information and way to confirm your student status) to: [email protected]
© 2026 All Rights Reserved.