Is Lurasidone Safe with Breastfeeding?

Want the latest research insights delivered straight to your inbox?
Published in: The Journal of Clinical Psychiatry, 2025; 86(4):25m15955
📌 Background
Managing maternal mental health during the postpartum period is often complex, especially when balancing effective treatment with the desire to continue breastfeeding.
While several second-generation antipsychotics (SGAs)—like olanzapine, quetiapine, and risperidone—have established lactation safety profiles, lurasidone (Latuda) has historically lacked direct human data. This gap often led clinicians to avoid prescribing lurasidone to breastfeeding mothers despite its favorable side effect and metabolic profile.
A 2025 study published in the Journal of Clinical Psychiatry now provides the first robust pharmacokinetic data assessing lurasidone transfer into human milk and its potential effects on breastfed infants.
🧪 Study Design
Design: Observational pharmacokinetic study (2022–2024)
Participants: 9 lactating mothers on lurasidone (20–80 mg/day)
Setting: InfantRisk Human Milk Biorepository
Method: Milk samples analyzed via liquid chromatography–mass spectrometry
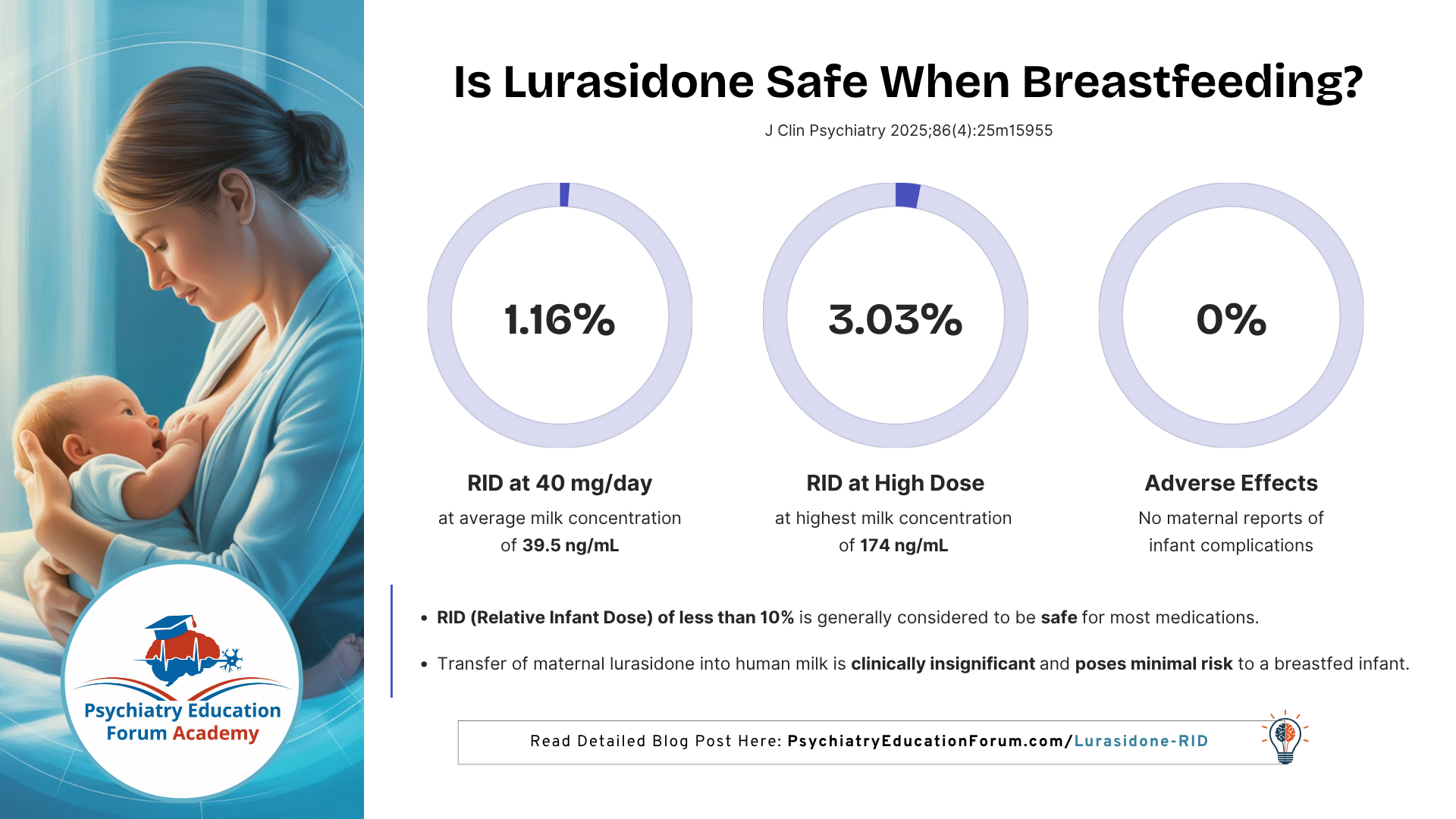
Primary goal: Quantify lurasidone concentration in milk and calculate relative infant dose (RID) — the percentage of maternal weight-adjusted dose received by the infant.
📊 Key Results:
Average lurasidone concentration in milk: 39.5 ng/mL (standardized to 40 mg/day dose)
Relative Infant Dose (RID):
Mean = 1.16%, far below the safety threshold of 10%
Maximum observed RID = 3.03%, still within acceptable limits
Clinical outcomes:
No adverse effects reported in any breastfed infants
No maternal discontinuations due to lactation concerns
💬 Clinical Context
Systematic reviews have long supported that SGAs generally have low transfer into breast milk and are rarely associated with adverse outcomes in infants. However, prior reviews classified lurasidone as lacking sufficient data for a confident safety rating.
This new study directly addresses that evidence gap:
✅ Drug transfer is minimal
✅ RID <10%, suggesting low infant exposure
✅ No observed adverse effects in any exposed infants
These findings align with FDA pharmacokinetic data showing that lurasidone’s high protein binding (99%) and short milk half-life limit its excretion into breast milk.
⚕️ Clinical Implications
Safe profile: At therapeutic doses (20–80 mg/day), lurasidone transfer into human milk appears clinically insignificant.
Infant safety: Exposure levels are well below thresholds associated with toxicity.
Clinical decision-making:
If lurasidone is the optimal antipsychotic for maternal stability, current evidence supports continuing it during breastfeeding.
Monitor infants for sedation, feeding issues, or developmental delay as a precaution, especially in premature or medically fragile infants.
⚖️ Comparison With Other SGAs
| Medication | Relative Infant Dose (RID%) | Adverse Infant Effects Reported |
|---|---|---|
| Lurasidone | 1.16 (max 3.03) | None observed |
| Olanzapine | 1.6–5.2 | Occasional sedation reported |
| Quetiapine | <1 | Rare |
| Risperidone | 2.3–4.7 | Minimal |
| Clozapine | Variable (avoid) | Risk of agranulocytosis |
📚 References
FDA Drug Label – Latuda (Lurasidone). Updated January 31, 2025.
Campbell LS, Datta P, Krutsch K. Balancing Mental Health and Breastfeeding: Evaluating the Transfer of Lurasidone Into Human Milk. J Clin Psychiatry. 2025;86(4):25m15955. doi:10.4088/JCP.25m15955. (article)
Uguz F. Second-Generation Antipsychotics During the Lactation Period: A Comparative Systematic Review on Infant Safety. J Clin Psychopharmacol. 2016;36(3):244–252.
Parikh T, Goyal D, Scarff JR, Lippmann S. Antipsychotic Drugs and Safety Concerns for Breast-Feeding Infants. South Med J. 2014;107(11):686–688.
Fortinguerra F, Clavenna A, Bonati M. Psychotropic Drug Use During Breastfeeding: A Review of the Evidence. Pediatrics. 2009;124(4):e547–e556.
FOR ACADEMY MEMBERS:
WOMEN'S MENTAL HEALTH:
PREGNANCY, POST-PARTUM & PERI-MENOPAUSE
-
Antipsychotics
- Antipsychotics During Pregnancy & Postpartum
- Antipsychotics: Rates of Placental Passage
- Antipsychotics of Choice during Lactation
-
Antidepressants
- Zuranolone FDA Approved for Postpartum Depression
- Antidepressants During Pregnancy & Postpartum
- Antidepressants of Choice during Lactation
- RE104 for Postpartum Depression (2025 Data)
-
Mood Stabilizers
- Preconception Planning: Preparing for Pregnancy
- Continue or Discontinue Medication during Pregnancy & Postpartum?
- Lithium: During Pregnancy & Postpartum
- Lithium: use during Lactation
- Valproate: During Pregnancy & Postpartum
- Lamotrigine: During Pregnancy & Postpartum
- Other Mood Stabilizers: During Pregnancy & Postpartum
- Other Mood Stabilizers: use during Lactation
-
Benzodiazepines
- Benzodiazepines: During Pregnancy & Postpartum
-
ADHD Medication
- ADHD Medication & Brestfeeding
-
Postpartum Onset OCD
- Postpartum Onset OCD
-
Postpartum Psychosis
- Postpartum Psychosis: Evaluation & Management
-
Perimenopausal Depression
- Perimenopausal Depression
-
PMDD
- PMDD
Women’s Mental Health (Pregnancy, Post-partum & Peri-menopause)
We continue to review and summarize clinically relevant research to support your daily practice.
INTERESTED IN ACCESS TO THIS & OTHER CLINICALLY RELEVANT LECTURE SERIES?
JOIN ACADEMY MEMBERSHIP:
This is a closed membership for medical professionals only.
- 400+ Clinically Relevant Chapters: Each chapter within these sections is of direct clinical relevance for your daily practice. (Table of Content)
- Journal Club: we will post the most recently published psychiatry articles relevant to your daily clinical practice. (Read Content)
- Clinical Case Discussion: Dr. Singh (Psychiatry) and Dr. Kaur (Family Medicine) discuss clinical cases to integrate the clinical cases from Psychiatry and Medicine. (Read Content)
- Monthly Insights: Gain access to our monthly sessions featuring the latest on recent publications, new medication approvals, FDA updates, and more. (Monthly Insights)
- Discussion Forum & Community: Connect with other medical professionals and discuss your difficult-to-treat clinical cases. (Academy Network)
- Goal: is to have all important clinically relevant topics in one place for ease of access.
DISCOUNTS AVAILABLE FOR: Residents & Students ONLY.
Email us your student information (program information and way to confirm your student status) to: [email protected]
© 2026 All Rights Reserved.