Esketamine Monotherapy Works—New Trial Validates FDA Approval


📌 Background
Until now, esketamine nasal spray had only been approved in combination with an oral antidepressant (OAD) for treatment-resistant depression (TRD). However, esketamine recently received FDA approval as monotherapy for TRD, providing a new option for patients who cannot tolerate or do not benefit from oral antidepressants.
This first placebo-controlled monotherapy trial validates that decision—providing strong evidence that esketamine alone can deliver rapid, robust antidepressant effects in patients with TRD, without needing adjunctive oral therapy.
- Janik A, Qiu X, Lane R, et al. Esketamine Monotherapy in Adults With Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. Published online July 02, 2025.
🧪 Study Design
Participants: 378 adults with TRD; no response to ≥2 prior antidepressants
Randomization: Esketamine 56 mg, 84 mg, or placebo—twice weekly for 4 weeks
Primary Outcome: Change in MADRS score at day 28
Key Secondary Outcome: Change in MADRS score at day 2 (24 hours post-dose)
Primary Efficacy Results: MADRS Change at Day 28
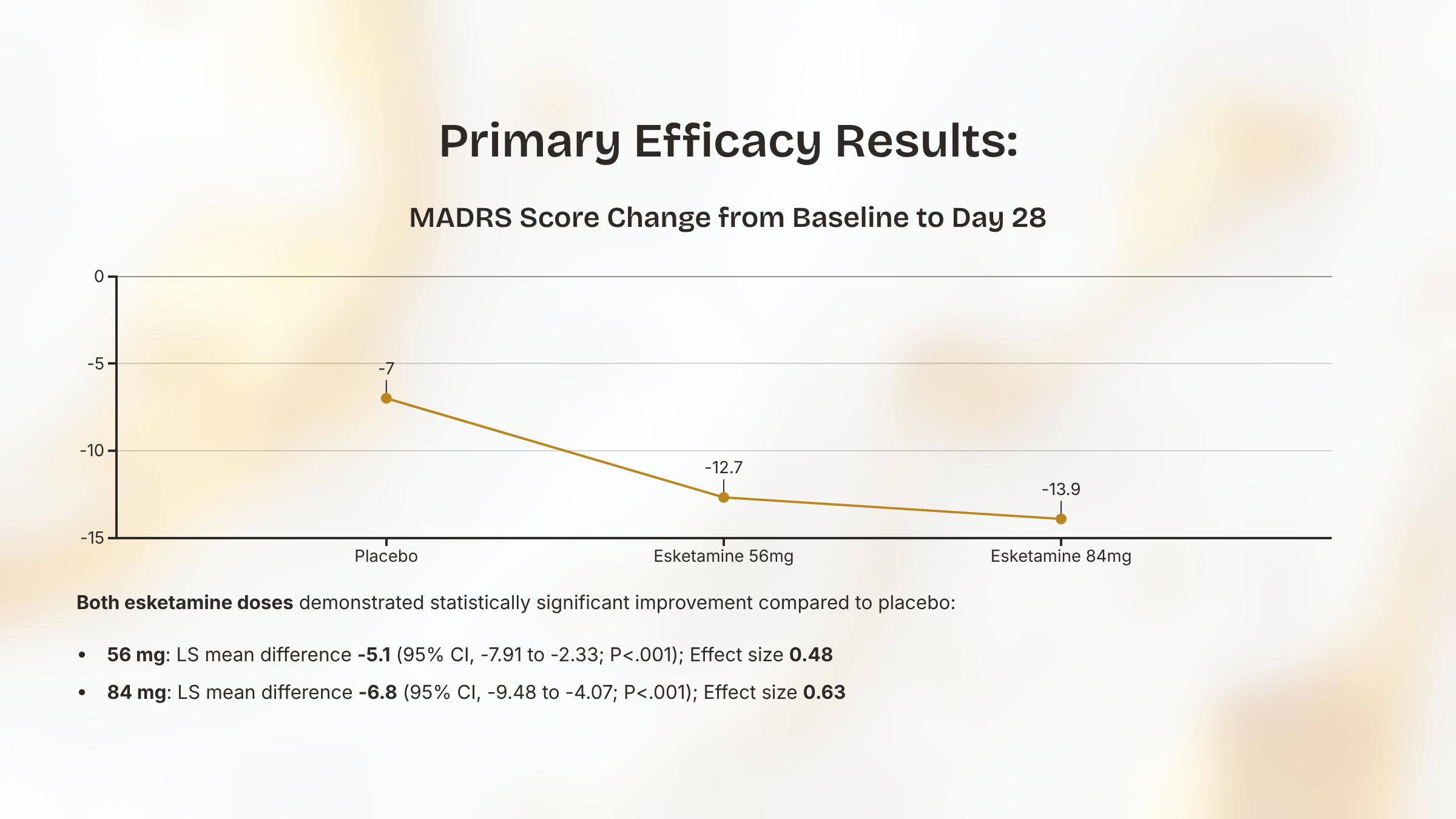
Mean MADRS score decreased (i.e., improved) from baseline to day 28 (primary efficacy endpoint).
Greater improvement observed in both esketamine dose groups (56 mg and 84 mg) compared to placebo.
Secondary Efficacy Results: MADRS Change at Day 2
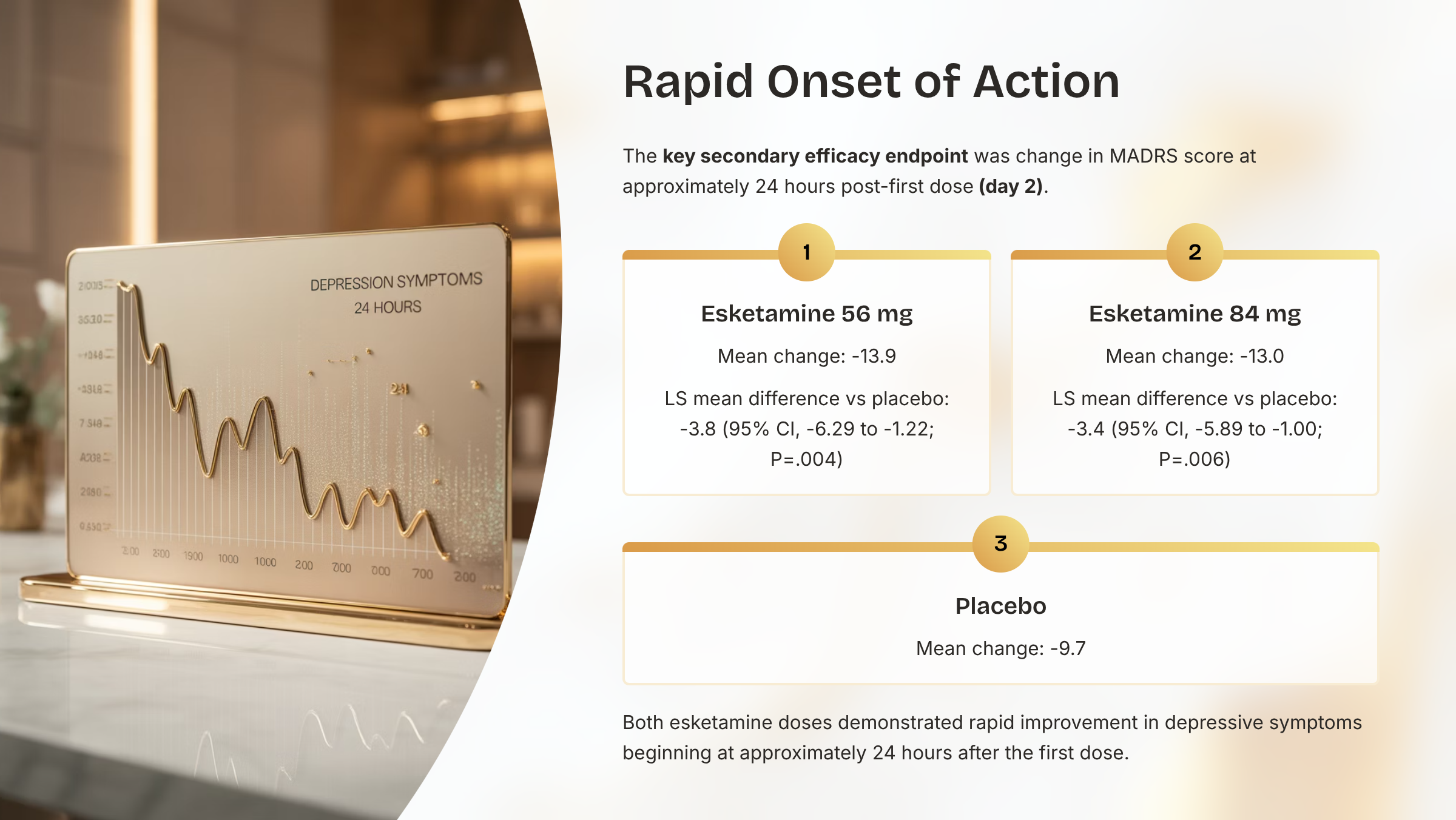
- Rapid improvement in depressive symptoms was observed approximately 24 hours after the first esketamine dose
Response & Remission Rates:

Response and remission rates were consistently higher in both esketamine groups compared to placebo at all double-blind assessment time points.
At day 28:
Response rates were approximately 2-fold higher in both esketamine dose groups vs placebo.
Remission rates were 2- to 3-fold higher in both esketamine dose groups vs placebo.
⚠️ Safety Profile:
Most common adverse events (>10% incidence) in esketamine-treated participants (combined doses) vs placebo:
Nausea: 24.8% (56/226) vs 8.4% (21/250)
Dissociation: 24.3% (55/226) vs 2.8% (7/250)
Dizziness: 21.7% (49/226) vs 7.2% (18/250)
Headache: 19.0% (43/226) vs 8.8% (22/250)
Incidence of adverse events was similar between the 56 mg and 84 mg groups (<5% difference for all events).
Timing of adverse events:
Majority occurred on dosing days:
Esketamine group: 85.2% (879 of 1032 events)
Placebo group: 65.9% (257 of 390 events)
Most resolved the same day:
Esketamine group: 89.8% (789 of 879 events)
Placebo group: 71.2% (183 of 257 events)
Nausea and vomiting were most common during early treatment:
Nausea: 65.6% (61 of 93) occurred in first 3 dosing sessions; 38.7% during first dose
Vomiting: 70.0% (14 of 20) occurred in first 3 sessions; 60.0% during first dose
Both symptoms attenuated in frequency with subsequent doses.
💬 Clinical Commentary
This trial supports the idea that targeting NMDA/glutamate systems can be effective even without adjunctive serotoninergic agents. Given the moderate-to-large effect size and early onset of benefit, esketamine monotherapy is now a legitimate option—especially for those unable or unwilling to take oral antidepressants.
Clinical Pearl: The response and remission rates here are similar to or slightly better than adjunctive trials—raising the question of whether monotherapy might reduce pharmacologic burden while still delivering benefit.
Caveats for Practice:
- REMS program enrollment is still required
- Monitoring for BP spikes and dissociation is essential during treatment
- Long-term safety and relapse prevention beyond the 32-day window are not established here
📚 Want to Learn More?
When the FDA approved Spravato as a monotherapy in January 2025, we published an in-depth clinical review with insights on dosing, patient selection, and workflow integration:
First Monotherapy FDA Approved for
Treatment-Resistant MDD
Spravato: First Monotherapy FDA Approved for Treatment-Resistant MDD
Want the latest research insights delivered straight to your inbox?
We continue to review and summarize clinically relevant research to support your daily practice.
INTERESTED IN ACCESS TO THIS & OTHER CLINICALLY RELEVANT LECTURE SERIES?
JOIN ACADEMY MEMBERSHIP:
This is a closed membership for medical professionals only.
- 400+ Clinically Relevant Chapters: Each chapter within these sections is of direct clinical relevance for your daily practice. (Table of Content)
- Journal Club: we will post the most recently published psychiatry articles relevant to your daily clinical practice. (Read Content)
- Clinical Case Discussion: Dr. Singh (Psychiatry) and Dr. Kaur (Family Medicine) discuss clinical cases to integrate the clinical cases from Psychiatry and Medicine. (Read Content)
- Monthly Insights: Gain access to our monthly sessions featuring the latest on recent publications, new medication approvals, FDA updates, and more. (Monthly Insights)
- Discussion Forum & Community: Connect with other medical professionals and discuss your difficult-to-treat clinical cases. (Academy Network)
- Goal: is to have all important clinically relevant topics in one place for ease of access.
DISCOUNTS AVAILABLE FOR: Residents & Students ONLY.
Email us your student information (program information and way to confirm your student status) to: [email protected]
© 2026 All Rights Reserved.